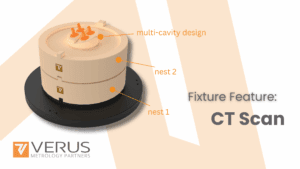
Medical device manufacturing requires extreme precision and reliability. From implants to diagnostic systems, every product must meet strict regulatory standards while performing flawlessly in clinical settings. In this environment, metrology is not just quality control—it underpins safety, compliance, and efficiency.
However, accurate dimensional inspection is challenging when dealing with complex geometries, delicate materials, and micro-scale features. Standard fixturing often falls short. Custom metrology fixtures, tailored to specific part geometries and inspection requirements, provide the consistency, repeatability, and efficiency MedTech manufacturers need.
This article explores how custom fixtures improve measurement integrity, regulatory compliance, and throughput, with practical insights and real-world examples.
Why Metrology Matters in MedTech
Dimensional accuracy in medical devices is directly tied to patient safety, compliance, and performance. Even small deviations in form or alignment can cause device failure, adverse outcomes, or regulatory issues.
Compliance requirements: Bodies such as the FDA (21 CFR Part 820) and ISO 13485 demand strict dimensional verification, measurement traceability, and documented inspection. During validation (IQ/OQ/PQ), manufacturers must demonstrate repeatable, reliable measurements. Failures here can delay approvals or trigger costly rework.
Unique challenges: MedTech parts may be micro-scale, thin-walled, flexible, or sensitive to load and contamination. Many have critical features—such as lumens or sealing interfaces—that must be measured without distortion. Inspection is often performed in cleanrooms under time pressure, where manual fixturing introduces error and inefficiency.
The Role of Fixtures in Inspection
Fixtures are essential for presenting parts consistently to measurement systems. They reduce operator variability, uncertainty, and alignment errors. In regulated industries, reliable fixturing is not optional—it is a core element of validated inspection processes.
Limitations of Generic Fixtures
-
Insufficient constraint: Generic solutions fit a wide range of parts but rarely secure MedTech components precisely. Loose fits or manual adjustments increase variation and undermine validation.
-
Operator dependency: Universal fixtures often require manual alignment, introducing human error—especially in high-throughput production.
-
Poor support for complex assemblies: Many devices involve multi-part systems or internal features, which generic fixtures cannot accommodate effectively.
-
Validation issues: Inconsistent fixturing leads to failed Gage R&R studies, delayed approvals, and workflow bottlenecks.
Conclusion: Generic fixtures often create more risk than they resolve.
The Value of Custom Metrology Fixtures
Custom fixtures eliminate variability by aligning design with part geometry, inspection requirements, and regulatory needs.
What Defines a Custom Fixture?
They are tailored to the component, incorporating:
-
Shaped nests for complex 3D profiles
-
Locating features for flexible/asymmetric parts
-
Datum references aligned with GD&T
-
Clearance for non-contact measurement
-
Modular elements for part families
Benefits
-
Improved R&R: Precisely locates parts, reducing variation by up to 50% or more.
-
Reduced cycle time: Faster loading, fewer reworks, smoother automation.
-
Better ergonomics: Simplifies handling, important in cleanroom environments.
-
Validation-ready: Provides detailed documentation to support audits.
-
Designed for compliance: Meets regulatory, mechanical, and process-specific needs.
Key Design Considerations
Effective fixtures must address material, geometry, process, and environment.
-
Material compatibility: Must protect delicate parts. Common options include PEEK, Delrin, ESD-safe polymers, and coated aluminium. Soft inserts prevent deformation.
-
Support for micro features: Fixtures must not obstruct or distort fine details. Precision machining or additive manufacturing may be required.
-
Manual vs automated use: Fixtures can be designed for ergonomic manual placement or robotic integration with datums, keys, or sensors.
-
Cleanroom suitability: Materials must be low-particulate, non-outgassing, and easy to clean, with smooth surfaces to avoid contamination traps.
-
Equipment integration: Fixtures must allow probe access, maintain stability, and align with software registration. Modular inserts provide flexibility.
Real-World Applications
Orthopaedic Implants
Challenge: Complex GD&T on polished metal parts.
Generic issue: V-blocks failed to constrain rotation, causing poor repeatability.
Custom solution: Multi-contact nest with functional datums and quick-release clamps.
Outcome: 60% R&R improvement, 40% faster setup, validation-ready.
Microfluidic Cartridges
Challenge: Thin-walled, transparent parts prone to distortion.
Generic issue: Manual clamps caused stress, distortion, and scrap.
Custom solution: Compliant nest with soft-touch ESD-safe inserts and magnetic detents.
Outcome: 90% reduction in handling damage, 2-micron repeatability, cleanroom compliant.
Catheter Assemblies
Challenge: Accurate length and profile inspection in high throughput.
Generic issue: Unsupported length caused sagging and misalignment.
Custom solution: Modular rail fixture with adjustable supports and datum features.
Outcome: Enabled automated optical inspection, halved changeover time.
Drug Delivery Devices
Challenge: Inspecting functional alignment in assemblies.
Generic issue: Separate clamping misrepresented real-world alignment.
Custom solution: Assembly simulation fixture with spring-loaded supports and datum alignment.
Outcome: ±5 micron repeatability, FDA validation supported.
Integrating Fixtures into Metrology Strategy
Custom fixturing’s true value comes when integrated into broader quality systems.
Measurement System Validation
Fixtures are critical in Gage R&R studies. Custom designs reduce variability and support validation reports. Any fixture change requires revalidation.
Integration with Turnkey Systems
Fixtures can be embedded in automated metrology cells, with kinematic mounts, programmed routines, and cross-platform compatibility.
Lifecycle Management
Like inspection equipment, fixtures require verification, calibration, and maintenance records to remain compliant.
Smart Manufacturing Alignment
Modern fixtures can embed sensors, integrate with digital twins, and support predictive quality analytics within Industry 4.0 frameworks.
Conclusion
In MedTech, precision and compliance are non-negotiable. Off-the-shelf fixtures rarely meet the demands of complex, delicate, or regulated components.
Custom metrology fixtures offer:
-
Higher repeatability and accuracy
-
Faster, more reliable inspection workflows
-
Compliance-ready documentation and traceability
-
Long-term alignment with validation, automation, and digital strategies
They are not secondary tools but critical enablers of scalable, validated, and efficient manufacturing.
For more detail, explore our Metrology Fixtures service page to see how tailored solutions can address your inspection challenges.