First Article Inspection & Process Verification
Get a comprehensive FAI report prepared by an experienced metrologist and tailored to your requirements
If you are introducing a new product to your production line, changing a manufacturing process, or for a range of other reasons, our First Article Inspection (FAI) and process verification services will help move your project to the next stage.
We specialise in providing full FAI services to manufacturers in the medical device industry, so we can deliver the standards and level of expertise that will meet your internal and compliance requirements.
First Article Inspection Process Overview
1. Preparation
The decisions you take in the early stages will determine the future direction of your project and the beneficial impact of your FAI report. Therefore, we can help with multiple elements in the preparation part of your project, including before you get to the FAI or process verification stage. Our consultation services will ensure your product design, quality control, and manufacturing teams get the metrology support they need, with expert advice during the development of your product and/or manufacturing process.
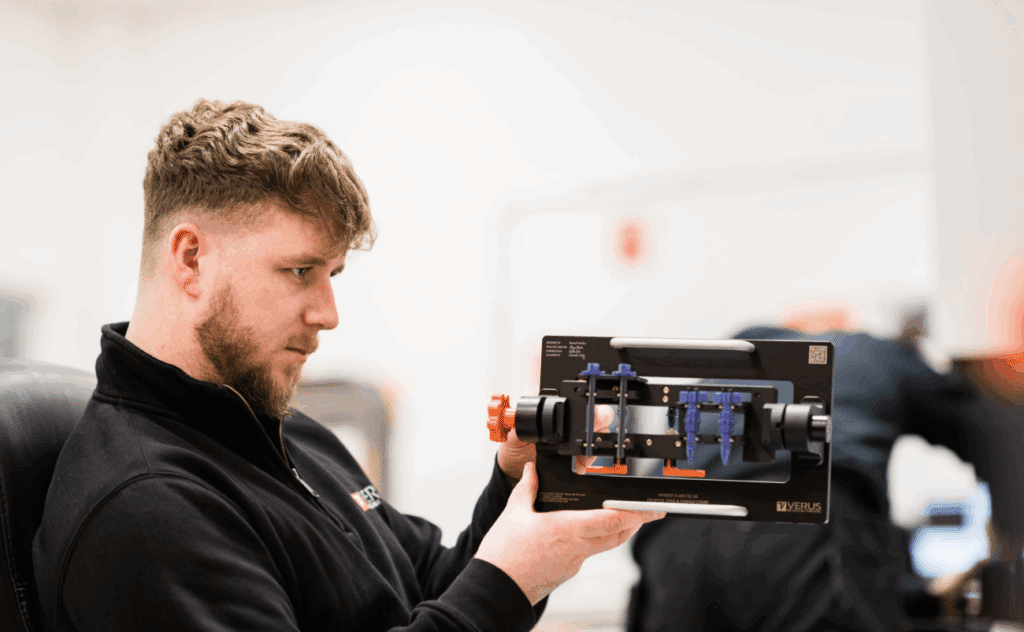
2. Inspection
Once you are ready to manufacture the first batch of products on your new or updated line, we’ll complete a detailed inspection. Our engineers will use our established FAI procedures to carry out the inspection, typically using a random selection of products from the initial manufactured batch.
The inspection will follow a pre-determined plan, and it will be completed on our advanced and fully calibrated inspection equipment operated by qualified metrology engineers.
3. First Article Inspection Report (FAIR)
We’ll then provide you with a comprehensive and impartial First Article Inspection Report outlining the results of the inspection. This will include whether the inspected parts match the design requirements according to the original specifications and engineering drawings.
This report will indicate either a pass or fail. In the case of a pass, the manufacturing of your products can continue, as you will have a high degree of assurance in the reliability of your manufacturing process.
Where the FAI report concludes the manufacturing process has failed, this indicates the discovery of variances between the inspected product and the product’s specification. Those variances will be outside the required tolerances.
In these FAI failure situations, our engineers will identify in as much detail as possible where the issues exist, so you can take remedial action.

First Article Inspection and Process Verification Services
- NPIs (new product introduction) – introducing a new medical device product to your production line
- Design change – verifying the manufacturing process following a design change to an existing product
- Manufacturing process change – re-verifying a manufacturing process after it has been modified or updated, such as where new tooling has been introduced, new equipment installed, or new materials sourced
- Factory relocations – our FAI service can verify the manufacturing process of a production line that has been moved to a new location
- Restarting a production line – if you have a product or production line that hasn’t been used for a long period of time, our FAI service can verify the manufacturing process
- Customer request – if you are a contract manufacturer of medical device products or components, your customer might request an FAI
All the above examples represent situations where FAIs are required to ensure your products remain in compliance with FDA, European, and other medical device regulations.

Creating an FAI Plan
Get expert support with all aspects of your FAI to ensure it is as successful and useful as possible. After all, the inspection and report generation components are only parts of an FAI project.
Creating a detailed plan for the FAI is also essential. We’ll ensure the plan for your project is fully tailored to your requirements, but common components of an FAI plan include:
- Information on the features and elements of the device, including materials and components
- Details of how the device will be inspected, both the objective and subjective elements
- The number of products that will be inspected in the first production run batch
- Gage R&R requirements
- Specific requirements and guidelines for the final FAI report
If you require a First Article Inspection report for a new product, updated product, updated manufacturing process, or for any other reason, get in touch with us today.

