Gage R&R
Is your measurement system repeatable and reproducible? A Gage R&R study will give you the data you need.
Ensure the integrity and accuracy of your measurement systems with our Gage Repeatability and Reproducibility (Gage R&R) services. Gage R&R can help with everything from compliance to product quality to waste minimisation to manufacturing process optimisation.
The Gage R&R study you receive will be comprehensive and unbiased, providing you with accurate information on whether your gage is acceptable, possibly acceptable, or not acceptable. In both latter instances, we can also help in identifying remedial steps that should be taken to bring the gage into an acceptable state.
At Verus Metrology, we are a reliable Gage R&R partner who will deliver on your specific requirements.
Gage R&R in the Medical Device Industry
Variation exists in all medical device manufacturing processes. This is why medical device components have tolerances, as tolerances take into account acceptable levels of variation. In the MedTech industry, tolerances can be very tight, but they still exist.
As variation exists, it is essential to understand the source of variation:.
- Is the variation caused by the manufactured product or component?
- Is the measurement system causing the variation?
If it is the latter, the products you are manufacturing could be within acceptable tolerances despite inspection results indicating otherwise. In other words, when the measurement system is the cause of variation, the data produced by your inspection processes is not reliable.
Poor data is a quality issue, but in the medical device industry, it also has patient safety implications. A Gage R&R study will help ensure the accuracy of your measurement data.
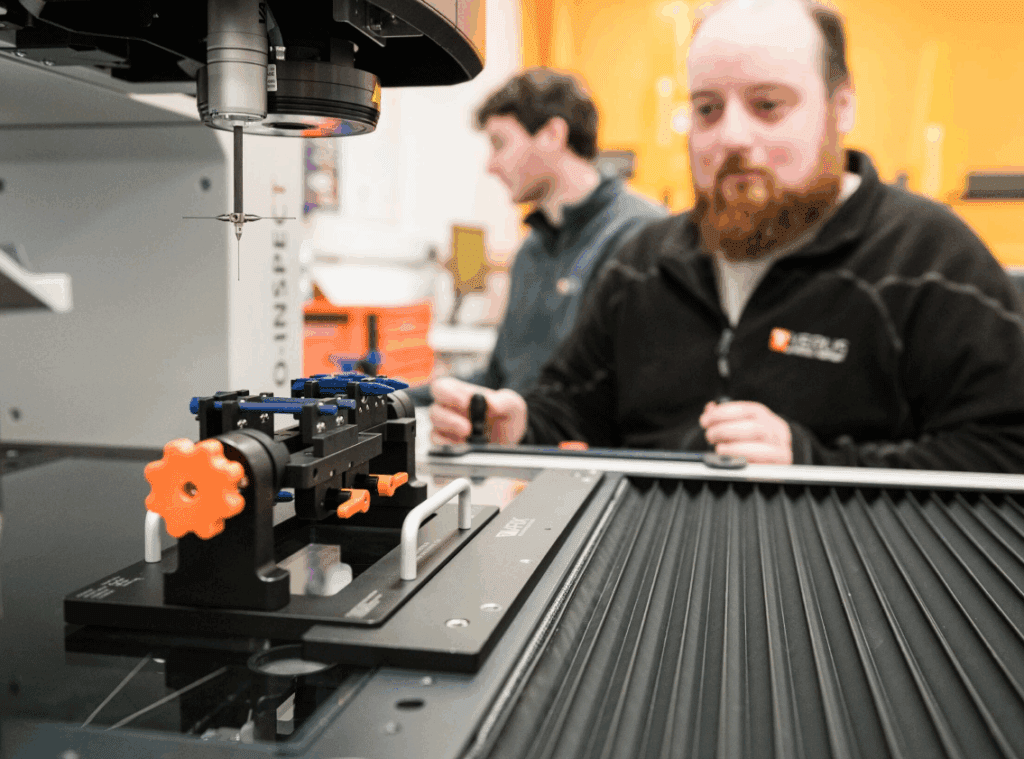
Repeatability & Reproducibility
The objectives of a Gage R&R study include:
- Evaluating the precision of your measurement process and the accuracy of your gauging instrument
- Understanding if your measurement system is adequate
- Distinguishing between variations in the product you are measuring, and variations caused by the inspection system (equipment, fixture, process, etc)
Your measurement system can include:
- CMMs
- Vision systems
- Metrology fixtures
Other equipment that is used to inspect and measure the medical device products and components that are produced on your production line.
This evaluation of gauging instrument accuracy is based on the repeatability and reproducibility of the measurements:
- Repeatability – are the same measurements produced multiple times by an operator using the same equipment and measuring the same part?
- Reproducibility – do different operators produce the same measurements when using the same equipment and measuring the same part?


Situations Where Gage R&R Studies Are Beneficial
- New metrology fixture development, especially during the qualification stage
- Evaluating other measurement and inspection equipment, including following repairs
- Comparing different inspection and measuring devices
- First article inspections (FAIs)
- Inspection process optimisation
- Metrology skills gap analysis assessments
- To enhance data-driven decision-making in relation to inspection and measurement processes and equipment
- Troubleshooting problems with your measurement system

